wawEBOC2iyRJfCHtkGYlQw1SRgBUdVhlO7HUlHUDnutc4S5PTK0wrMEMsgNpoAFus4Ew
A Certificate of Analysis (CoA) is a document that does exactly that - it summarizes all the tests performed on a product and gives the needed reassurance to the customers. It is therefore an essential document for most companies in the chemical and food sector.

Weighty Matters Australian Food Industry Launches World's Least
Through long-established industry practice, an ingredient supplier typically provides customers with a Certificate of Analysis (COA) with each delivered batch. The goal of this publication is to present recommendations on the content and format of such COAs including: specifications for identity and, as applicable, specifications on other.

FileNCI Visuals Food Taco.jpg Wikimedia Commons
6 7 8 1 Microbiological Testing by Industry of Ready-to-Eat Foods Under FDA's Jurisdiction for Pathogens 2 (or Appropriate Indicator Organisms): Verification of Preventive Controls. 1 . 3 ADOPTED 22 APRIL 2021, WASHINGTON, DC 4 NATIONAL ADVISORY COMMITTEE ON MICROBIOLOGICAL CRITERIA FOR FOODS 5 . NACMCF Executive Secretariat*, U.S. Department of Agriculture, Food Safety

COA Hosts Cancer Care Teams, Advocates, and Stakeholders for Largest
Contrastingly, a Certificate of Analysis (CoA) is a document, usually provided by an external testing agency or third-party laboratories, verifying that a product has undergone detailed testing and met the specified identification and performance criteria. Industries like pharmaceuticals, food products, and chemicals frequently utilize CoAs.

National Food Authority Gensan General Santos City
Certificates of analysis (COAs) hold the key to improving quality and productivity and focusing on continuous improvement rather than just covering your assets. Issues with Paper COAs COAs provide a snapshot into a supplier's production process.

Shop Online Herbal Tea, Herbal Medicine, Slim Tea, Supplements, Oils
A Certificate of Analysis (COA) is a document that manufacturers produce that verifies the product they manufactured conforms to their customer's requirements. It is important for the customers to know that the product they are receiving adheres to their specific parameters and targets, and to ensure that it meets their needs.
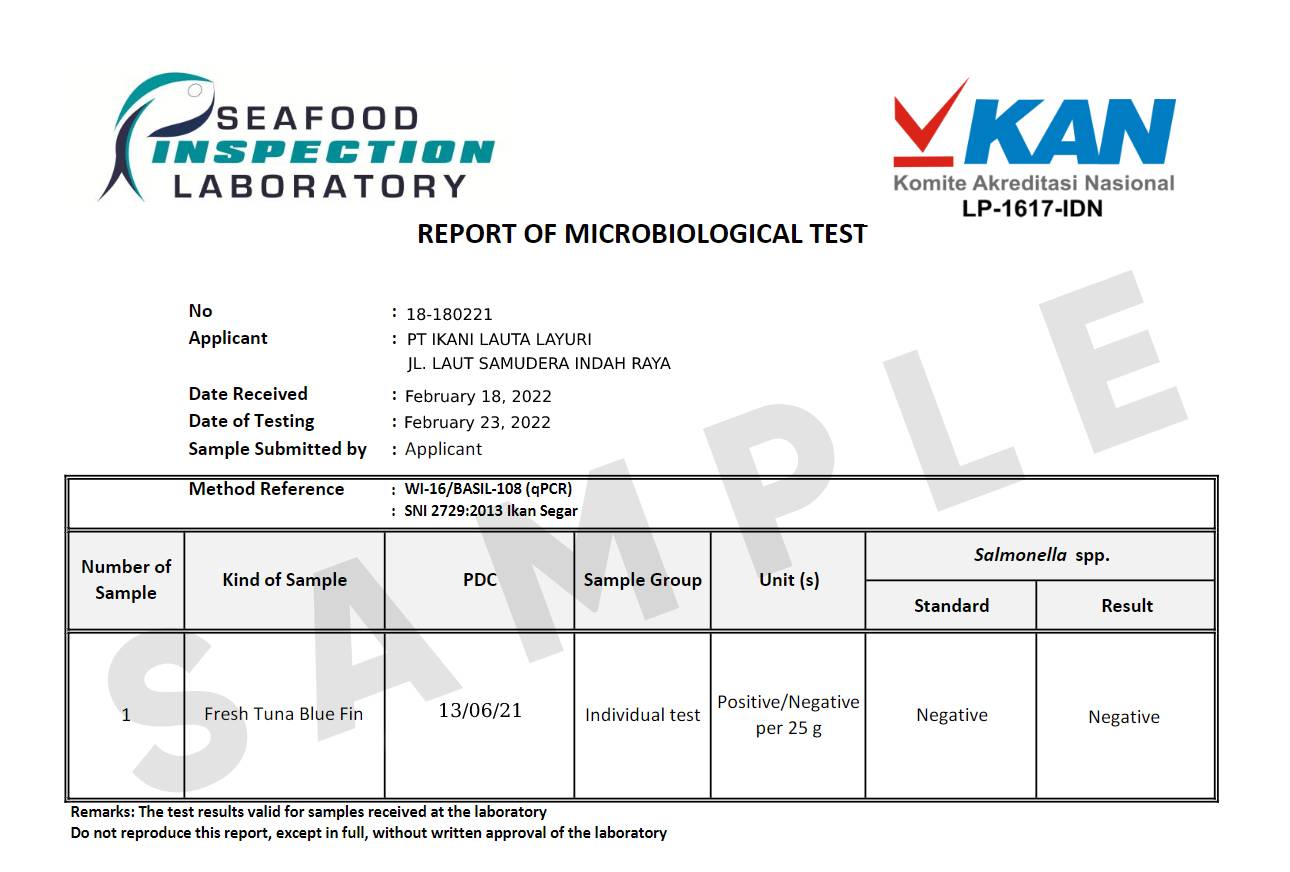
COA
NACMCF RTETesting_MainText_Final_12 July 2021 LP_cleancopyFSISwebsite.docx . 1 of . 63 . 1 Microbiological Testing by Industry of Ready-to-Eat Foods Under FDA's Jurisdiction for Pathogens 2 (or Appropriate Indicator Organisms): Verification of Preventive Controls 3 ADOPTED 22 APRIL 2021, WASHINGTON, DC 4 2018-2020 NATIONAL ADVISORY COMMITTEE ON MICROBIOLOGICAL CRITERIA FOR FOODS

Best Practices & Remedies to Avoid COA Disallowances
Evaluate general supplier information and a sample of the raw material against your food quality checklist. In this process, test the raw material for its composition, purity, and potential presence of microbiological contamination. In addition, you can perform a test run at your facility. As a second step, evaluate the supplier's.

Therapy Company Coa Secures 3 Million
A Certificate of Analysis (also known as a COA) is a document issued by a quality assurance or third-party testing laboratory that provides detailed information about the composition and quality of a product. It typically includes data on key components, concentrations, purity, heavy metals, bacterial count, and other relevant metrics.

Angel Indian Organic Cassava Flour, 25 / 50 at Rs 85/kg in Erode ID
CAPA and Root Cause Analysis for the Food Industry. A thorough and effective CAPA can provide many benefits such as providing long-term solutions, preventing recurrences, fostering continuous improvement, improving customer satisfaction, improving profitability, and having the ability to influence FDA and FSMA inspections.
File"Rules and Regulations...Threshing Committee of the U.S. Food
A certificate of analysis (COA) is a formal laboratory-prepared document that details the results of (and sometimes the specifications and analytical methods for) one or more laboratory analyses, signed—manually or electronically—by an authorized representative of the entity conducting the analyses. This document gives assurances to the recipient that the analyzed item is what it is.

COA fckorea
A Certificate of Analysis (COA) serves as a vital assurance in the ingredient industry, providing a comprehensive snapshot of a product's quality and compliance. Essentially, it's a document offered by manufacturers to affirm that their products meet the predefined standards and specifications agreed upon with customers.

COA
Well, a Certificate of Analysis (CoA) is an important document provided with a range of manufactured products like food, chemicals, research products, and pharmaceutical products. It is important for the customers to know that the product they are receiving adheres to their specific parameters and targets, and to ensure that it meets their.

What is a CBD Certificate of Analysis (COA) (And How to Read It
What is Certificate of analysis (COA)? COA is a document proving and explaining that certain laboratory tests have been carried out on products before export. It could be used to show that the food laboratory has already tested your food samples in order to ensure it is wholesome & fit for human consumption.

Organic Food Focus
A Certificate of Analysis (CoA) is a general term used across all manufacturing and commodity industries. For example, CoAs apply to manufactured car parts that are passed to an assembly plant as well as fresh food delivered to grocery stores or restaurants.

Baya Food Products
What is a Certificate of Analysis (COA)? A Certificate of Analysis (COA) is an official document generated by a laboratory outlining the outcomes of one or more laboratory tests. It may also include specifications and analytical methods, and it is signed by an authorized representative through manual or electronic means.